Efficacy
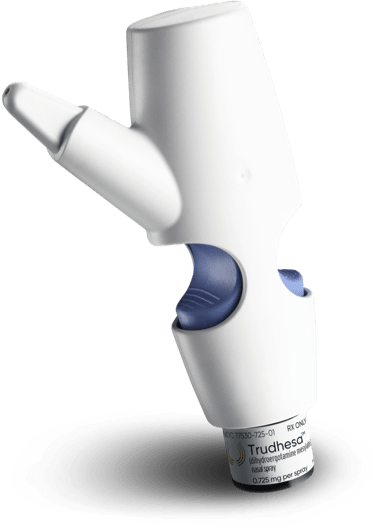
Benefits of DHE and Precision Olfactory Delivery (POD®) technology1,2
In 4 randomized, double-blind, placebo-controlled studies (N=605) of a traditional dihydroergotamine mesylate (DHE) nasal spray product, 30% to 61% of patients had mild or no pain at 2 hours1

Trudhesa® with advanced POD technology consistently delivered DHE without the initial peak concentration of intravenous (IV) DHE2

Trudhesa demonstrated rapid, sustained, consistent relief, even when taken late into an attack2,3*
*Based on a phase 3, open-label, safety study evaluating exploratory efficacy outcomes and post hoc analyses.2
The clinical efficacy studies of a traditional DHE nasal spray product1
A traditional DHE nasal spray product was evaluated in 4 randomized, double-blind, placebo-controlled studies.1
Percentage of patients who reported mild or no pain† following a single treatment1
N=105
N=98 23%
N=103
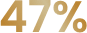
N=102 33%
N=50
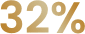
N=50 20%
N=47
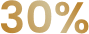
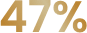
N=50 20%
†Headache response was defined as a reduction in headache severity to mild or no pain. Headache response was based on pain intensity as interpreted by the patient using a 4-point or 5-point pain intensity scale. Patients treated a moderate to severe migraine headache with a single dose of study medication and assessed pain severity over the 24 hours following treatment. Headache response was determined 0.5, 1, 2, 3, and 4 hours after dosing. Although rescue medication was allowed in all 4 studies, patients were instructed not to use it during the 4-hour observation period.1
‡P<.001; §P<.01.1
Results from the Trudhesa comparative bioavailability study

Consistently delivered DHE via POD without the initial peak concentration of IV DHE4
Trudhesa reached effective plasma concentrations in 30 minutes, steadily for well-established tolerability4

Results from the Trudhesa safety study
With Trudhesa, patients can achieve rapid, sustained, consistent relief even when taken late into an attack.2,3,||
A total of 5099 doses of Trudhesa were self-administered by 354 patients over the first 24 weeks of the study to treat 4515 migraine attacks.2
||Based on exploratory end points and their post hoc analyses of patient-reported efficacy data.2

Rapid pain relief delivered
Pain Relief Post-Dose
Ad hoc analysis of first migraine attack treated with Trudhesa2
Actor portrayal.
Study patients using their best usual care reported a 30% rate of response at 2 hours.3

Sustained pain freedom delivered
Sustained pain freedom is defined as the percentage of patients who self-reported no other headache at 24 and 48 hours post-Trudhesa administration.3
Of the 4257 migraine attacks treated during the 24-week study, where Trudhesa was used first
¶Data are self-reported. Excludes migraine attacks that started at baseline and ended in Weeks 1-24 with acute medication use in both periods. These analyses were exploratory in nature and no statistics were done.3

Consistent results delivered
Trudhesa provides consistent absorption and reliable relief by delivering proven DHE to the upper nasal space via POD.1,4

Pain freedom delivered late in an attack
Patient-reported results#
As part of the Trudhesa safety study, patients kept a daily e-diary and completed questionnaires about Trudhesa as compared to their best usual care.2**

Actor portrayal.
# Based on results from a poststudy questionnaire, which asked 354 people to rate their experience with Trudhesa against their best usual care, which consisted of their optimal migraine medications established by the patient or healthcare professional. Results were patient-reported and not validated.2,7
**Best usual care = optimal migraine medications established by the patient or healthcare professional. This comprised (sometimes more than one of) the following: acetaminophen, non-steroidal anti-inflammatory drug (NSAID), triptan, combination analgesic, opioid, barbiturate, or ergot.2,7

Traditional DHE nasal spray
Studies 1 and 2
In studies 1 and 2, doses of 2 mg or 3 mg DHE treatment were assessed. For a single treatment, the higher dose showed no advantage over the lower dose. A 4-point pain intensity scale was used to evaluate responses.1
Studies 3 and 4
In studies 3 and 4, a total dose of 2 mg was compared with placebo. A 5-point scale was used that included pain response.1
Trudhesa bioavailability study
This was a phase 1, open-label, randomized, single-dose, 3-period, 3-way crossover study. Subjects were screened for up to 21 days before randomization into 1 of 6 sequences, dictating the order of the 3 treatments to produce a balanced crossover, with a 7-day washout between each treatment.*
Subjects:
A total of 38 healthy volunteers aged 18 to 55 years, with no significant medical history were enrolled in the phase 1 study.
End points:
The safety, tolerability, and bioavailability of DHE following a single-dose administration of Trudhesa 1.45 mg was compared with IV DHE 1.0 mg and DHE nasal spray 2.0 mg in healthy adult subjects.
*Pretreatment with an antiemetic (metoclopramide 10 mg, delivered by slow IV push over 1 to 2 minutes, 5 to 10 minutes prior to DHE dosing) was given in all 3 treatment arms.
Trudhesa safety study
Phase 3, open-label safety, tolerability, and exploratory efficacy study.
- 4515 migraine attacks were treated with acute use of Trudhesa compared to best usual care* for up to 52 weeks
- Full safety set (FSS) = 354 patients who took ≥1 dose of Trudhesa
- Primary safety set (PSS) = 185 patients who took ≥2 doses of Trudhesa per 28-day period during the 24-week treatment period
- 28-week extension = 73 patients continued with treatment to 52 weeks total
Patient baseline characteristics (N=354)2:
- Mean age:
41.3 years - Body mass index (BMI):
30.4 kg/m² - Duration of migraine history:
19.5 years - Migraine attacks during the 28-day screening period:
4.6 - Gastrointestinal (GI) disorders:
38.4% - Gastroesophageal reflux disease (GERD):
20.3% - Cardiac disorders:
5.1%†
Primary end points2,3:
- Serious and nonserious treatment-emergent adverse events
- Change in nasal mucosa and change in olfactory function
Secondary end points:
- Changes in vital signs, physical examinations, 12-lead electrocardiogram (ECG), and laboratory evaluations
Exploratory end points were self-reported efficacy outcomes, including2*:
- Pain freedom, MBS freedom at 2, >2 to 4, and >4 hours
- Pain relief at 2 hours
- Recurrence of migraine pain through 24 and 48 hours
*Baseline consisted of acute treatment for migraine, or best usual care established by the patient or healthcare professional and was self- administered to treat migraine attacks in the 28-day prestudy period, with response comprising baseline.2
†Patients were excluded if they had a history of CV events or presented with significant risk factors for CV disease. Patients with a history of hypertension could enroll if hypertension was stable and well-controlled on current therapies for >6 months, provided no other risks were present.2
References: 1. Trudhesa. Prescribing information. Impel Pharmaceuticals; 2021. 2. Smith TR, Winner P, Aurora SK, Jeleva M, Hocevar-Trnka J, Shrewsbury SB. STOP 301: a phase 3, open-label study of safety, tolerability, and exploratory efficacy of INP104, Precision Olfactory Delivery (POD®) of dihydroergotamine mesylate, over 24/52 weeks in acute treatment of migraine attacks in adult patients. Headache. 2021;61(8):1214-1226. 3. Data on File. Impel Pharmaceuticals. 2020. 4. Shrewsbury SB, Jeleva M, Satterly KH, Lickliter J, Hoekman J. STOP 101: a phase 1, randomized, open-label, comparative bioavailability study of INP104, dihydroergotamine mesylate (DHE) administered intranasally by a I123 Precision Olfactory Delivery (POD®) Device, in healthy adult subjects. Headache. 2019;59(3):394-409. 5. Tepper SJ, Ailani J, Shrewsbury SB, Aurora SK. Recurrence rates for INP104 for the acute treatment of migraine: results from the phase 3 STOP 301 study. Poster presented at: American Headache Society Virtual Annual Scientific Meeting, June 3-6, 2021. 6. Lipton RB, Nye BL, Hirman J, Shrewsbury SB, Aurora SK. Treatment consistency across multiple migraine attacks: results from the phase 3 open-label STOP 301 study. Poster presented at: American Headache Society Virtual Annual Scientific Meeting, June 3-6, 2021. 7. Shrewsbury SB, Joekman J, Jeleva M. Patient acceptability of a novel upper nasal delivery system for dihydroergotamine mesylate using the Precision Olfactory Delivery (POD®) device – results from the open-label STOP 301 trial. Poster presented at: American Headache Society Virtual Annual Scientific Meeting, June 3-6, 2021.